

Ultralow Frequency Transmitted Sound Imaging INVESTOR PRESENTATION January 2024 1 Filed by GigCapital5, Inc. Pursuant to Rule 425 under the Securities Act of 1933, as amended, and deemed filed pursuant to Rule 14a-12 under the Securities Exchange Act of 1934, as amended Subject Company: QT Imaging, Inc. Commission File No.: 333-269760

Disclaimer ABOUT THIS PRESENTATION This investor presentation (this “Presentation”) is provided for informational purposes only and has been prepared to assist interested parties in making their own evaluation with respect to the proposed business combination (the “Proposed Business Combination”) between QT Imaging, Inc. (“QT Imaging™”) and GigCapital5, Inc. (“GigCapital5”) and for no other purpose. The information contained herein does not purport to be all-inclusive or to contain all of the information that may be required to make a full analysis of QT Imaging or the Proposed Business Combination, and none of GigCapital5, QT Imaging, and William Blair & Co. LLC (“William Blair”), or their respective directors, officers, employees, agents, advisors or affiliates makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation, which has not been verified and is subject to change at any time. Viewers of this Presentation should each make their own evaluation of QT Imaging, the Proposed Business Combination and of the relevance and accuracy of the information and should make such other investigations as they deem necessary. To the fullest extent permitted by law, no responsibility or liability whatsoever is accepted by GigCapital5, QT Imaging, or their respective directors, officers, employees, agents, advisors or affiliates for any loss howsoever arising, directly or indirectly, from any use of this Presentation or such information or opinions contained herein or otherwise arising in connection herewith. This Presentation does not constitute (i) a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the Proposed Business Combination or (ii) an offer to sell, a solicitation of an offer to buy, or a recommendation to purchase any security of GigCapital5, QT Imaging, or any of their respective affiliates, nor shall there be any sale, issuance or transfer of securities in any jurisdiction where, or to any person to whom, such offer, solicitation or sale would be unlawful. You should not construe the contents of this Presentation as legal, tax, accounting or investment advice or a recommendation. You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this Presentation, you confirm that you are not relying upon the information contained herein to make any decision. Any offer to sell securities will be made either (a) pursuant to a definitive subscription agreement (a “Subscription Agreement”) for GigCapital5 and will be made in reliance on an exemption from registration under the Securities Act of 1933, as amended (the “Securities Act”), for offers and sales of securities that do not involve a public offering, or (b) with respect to the Proposed Business Combination, by means of a joint proxy statement/prospectus of GigCapital5 (the “GigCapital5 proxy statement/prospectus”) that complies with applicable rules and regulations promulgated under the Securities Act and the Securities Exchange Act of 1934, as amended (the “Exchange Act”). GigCapital5, QT Imaging and their respective affiliates reserve the right to withdraw or amend for any reason any offering and to reject any Subscription Agreement for any reason. Any public offering of securities shall be made only by means of a prospectus meeting the requirements of the Securities Act. On June 6, 2017, the U.S. Food and Drug Administration ("FDA") in response to QT Imaging’s Section 510(k) Summary of Safety and Effectiveness premarket notification under the Food, Drug and Cosmetic Act, determined that the QT Breast Scanner is substantially equivalent to the predicate device. Our use of the words “safe”, “safety”, “effectiveness”, and “efficacy” in relation to the QT Breast Scanner in this Presentation, in the proxy statement/prospectus and all other QT Imaging related documents is limited to the context of the Section 510(K) Summary of Safety and Effectiveness that was reviewed and responded to by the FDA. Copyright © 2023 QT Imaging, Inc. All Rights Reserved.

Copyright © 2024 QT Imaging, Inc. All Rights Reserved. Disclaimer – Cont. Industry and Market Data Industry and Market data used in this Presentation have been obtained from public sources and third-party industry publications. Such data has not been independently verified by QT Imaging or GigCapital5, and although such data is believed to be reliable, QT Imaging and GigCapital5 cannot assure its accuracy or completeness. The data is also subject to change. You are cautioned not to give undue weight to such industry and market data. Certain information contained in this Presentation relates to or is based on QT Imaging’s own internal estimates and research. While QT Imaging believes its internal research is reliable, such research may not have been or has not been verified by any independent source and none of GigCapital5, QT Imaging or any of their respective affiliates nor any of its or their control persons, officers, directors, employees or representatives make any representation or warranty with respect to the accuracy of such research and information. This Presentation does not purport to be all inclusive or to contain all of the information that may be required to make a full analysis of QT Imaging, GigCapital5 or the Proposed Business Combination. Readers of this Presentation should make their own evaluation of QT Imaging, GigCapital5 and the Proposed Business Combination, and make such other investigations as they deem necessary. Forward Looking Statements This Presentation includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. The expectations, estimates, and projections of the businesses of GigCapital5 and QT Imaging may differ from their actual results and consequently, you should not rely on these forward-looking statements as predictions of future events. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions are intended to identify such forward-looking statements. These forward‑looking statements include, without limitation, expectations of the management of QT Imaging with respect to the business and prospects of QT Imaging and the QTscan® and other products of QT Imaging, the benefits of the Proposed Business Combination, the plans, expectations and intentions of QT Imaging and GigCapital5 and the future performance of QT Imaging, including the anticipated impact of the Proposed Business Combination on this performance. These forward-looking statements are (i) based on various assumptions, whether or not identified in this Presentation, and on the current expectations of management of QT Imaging, (ii) not predictions of actual performance, (iii) provided for illustrative purposes only and (iv) not intended to serve as, and must not be relied on by any investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability or any representation by any person that the forward-looking statements set forth in this Presentation or the results contemplated thereby will be achieved.

Copyright © 2024 QT Imaging, Inc. All Rights Reserved. Disclaimer – Cont. Forward Looking Statements – Cont. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. Many actual events and circumstances are beyond the control of the parties to the Proposed Business Combination and are difficult to predict. These forward-looking statements involve significant risks and uncertainties that could cause the actual results to differ materially from the expected results, including, but not limited to: (1) the ability of GigCapital5, QT Imaging and the surviving company to issue equity or equity-linked securities in connection with the Proposed Business Combination or in the future, (2) the outcome of any legal proceedings that may be instituted against the parties following the announcement of the Proposed Business Combination and the definitive agreements with respect thereto; (3) the inability to complete the Proposed Business Combination, including the risk that any regulatory approvals or the SEC’s declaration of the effectiveness of the GigCapital5 proxy statement/prospectus are not obtained, are delayed or are subject to unanticipated conditions that could adversely affect the surviving company or the expected benefits of the Proposed Business Combination or due to failure to obtain approval of the stockholders of GigCapital5 and QT Imaging or other conditions to closing; (4) the amount of redemption requests made by GigCapital5’s stockholders; (5) the impact of the COVID-19 pandemic on (x) the parties’ ability to consummate the Proposed Business Combination and (y) the business of QT Imaging and the surviving company; (6) the receipt of an unsolicited offer from another party for an alternative business transaction that could interfere with the Proposed Business Combination; (7) the inability to obtain or maintain the listing of the surviving company’s common stock on the Nasdaq or any other national stock exchange following the Proposed Business Combination; (8) the risk that the Proposed Business Combination disrupts current plans and operations as a result of the announcement and consummation of the Proposed Business Combination; (9) the ability to recognize the anticipated benefits of the Proposed Business Combination, which may be affected by, among other things, competition, the ability of the surviving company to grow and manage growth profitably and retain its key employees; (10) costs related to the Proposed Business Combination; (11) changes in applicable laws or regulations; (12) the demand for QT Imaging’s and the surviving company’s services together with the possibility that QT Imaging or the surviving company may be adversely affected by other economic, business, and/or competitive factors; (13) risks and uncertainties related to QT Imaging’s business, including, but not limited to, the ability of QT Imaging to increase sales of its output products in accordance with its plan; (14) risks related to the rollout of QT Imaging’s business and the timing of expected business milestones; (15) the effects of competition on QT Imaging’s business; (16) changes in domestic and foreign business, market, financial, political, and legal conditions; and (17) other risks and uncertainties included at the end of the Presentation or in (x) the “Risk Factors” sections of the most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q filed with the SEC by GigCapital5 and (y) other documents filed or to be filed with the SEC by GigCapital5. The foregoing list of factors is not exclusive.

Copyright © 2024 QT Imaging, Inc. All Rights Reserved. Disclaimer – Cont. Forward Looking Statements – Cont. If any of these risks, uncertainties or other factors materialize or QT Imaging’s assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that neither GigCapital5 nor QT Imaging presently know or that they currently believe are immaterial that could also cause actual results to differ from those contained in the forward-looking statements contained in this Presentation. In addition, forward looking statements in this Presentation speak only as of the date of this Presentation. While GigCapital5 and QT Imaging may elect to update these forward-looking statements at some point in the future GigCapital5 and QT Imaging do not undertake or accept any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements to reflect any change in their expectations or any change in events, conditions, or circumstances on which any such statement is based and specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing GigCapital5’s and QT Imaging’s assessments as of any date subsequent to the date of this Presentation. Accordingly, undue reliance should not be placed upon these forward-looking statements. Information and opinions expressed in this Presentation were obtained from sources believed to be reliable and in good faith, and no representation or warranty, express or implied, is made as to the accuracy or completeness thereof. This Presentation contains preliminary information only, is subject to change at any time and is not, and should not be assumed to be, complete or constitute all the information necessary to adequately make an informed decision regarding your engagement with GigCapital5 or QT Imaging. Use Of Projections This Presentation contains projected operational information with respect to QT Imaging, which constitutes forward-looking information for illustrative purposes only. It should not be relied upon as indicative of future results. See “Forward-Looking Statements” above. QT Imaging has a limited operating history, has not generated significant revenues, has an unproven business model, and may face intense competition that makes it impossible to reliably predict future growth and operating results.

Copyright © 2024 QT Imaging, Inc. All Rights Reserved. Disclaimer – Cont. Trademark And Intellectual Property QT Imaging and GigCapital5 own or have proprietary rights to various trademarks, service marks and trade names used in this Presentation that are important to their respective businesses, many of which are registered under applicable intellectual property laws. This Presentation also contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade names or products in this Presentation is not intended to, and does not, imply a relationship with QT Imaging or GigCapital5, or an endorsement or sponsorship by or of QT Imaging or GigCapital5. Solely for convenience, trademarks, trade names and service marks referred to in this presentation may appear without the ®, ™ or SM symbols, but such references are not intended to indicate, in any way, that QT Imaging or GigCapital5 or any third party, as applicable, will not assert, to the fullest extent permitted under applicable law, its rights or the right of the applicable licensor to these trademarks, trade names and service marks. Participants in the Solicitation GigCapital5, QT Imaging, and their respective directors, executive officers and other members of their management and employees, under SEC rules, may be deemed to be participants in the solicitation of proxies of GigCapital5 stockholders in connection with the Proposed Business Combination. Investors and security holders may obtain more detailed information regarding the names, affiliations and interests of GigCapital5’s directors and officers in its Annual Report on Form 10-K for the fiscal year ended December 31, 2022, which was filed with the SEC on March 31, 2023 (the “GigCapital5 Annual Report”). Information regarding the persons who may, under SEC rules, be deemed participants in the solicitation of proxies to GigCapital5’s stockholders in connection with the Proposed Business Combination will be set forth in the GigCapital5 proxy statement/prospectus for the Proposed Business Combination when available. Information concerning the interests of GigCapital5’s and QT Imaging’s equity holders and participants in the solicitation, which may, in some cases, be different than those of GigCapital5’s and QT Imaging’s equity holders generally, will be set forth in the GigCapital5 proxy statement/prospectus relating to the Proposed Business Combination when it becomes available. Stockholders, potential investors and other interested persons should read the proxy statement/prospectus carefully when it becomes available before making any voting or investment decisions. You may obtain free copies of these documents, once available, at the SEC’s website at www.sec.gov.

Copyright © 2024 QT Imaging, Inc. All Rights Reserved. Disclaimer – Cont. Additional Information and Where To Find It In connection with the Proposed Business Combination, it is intended that GigCapital5 will prepare the GigCapital5 proxy statement/prospectus in a registration statement on Form S-4 (the “Registration Statement”) to be filed with the SEC. The Registration Statement will include a preliminary proxy statement, and following review by the SEC, a definitive proxy statement to be mailed to GigCapital5’s stockholders in connection with GigCapital5’s solicitation of proxies for the vote by GigCapital5’s stockholders with respect to the Proposed Business Combination and other matters described in the Registration Statement, and a prospectus relating to the offer of the securities to be issued by GigCapital5 in connection with the Proposed Business Combination. GigCapital5 and QT Imaging urge investors and other interested persons to read, when available, the Registration Statement, as well as other documents filed with the SEC, because these documents will contain important information about QT Imaging, GigCapital5 and the Proposed Business Combination. When available, the definitive GigCapital5 proxy statement/prospectus and other relevant materials for the Proposed Business Combination will be mailed to stockholders of GigCapital5 as of a record date to be established for voting on the Proposed Business Combination. Such persons can also read the GigCapital5 Annual Report for a description of the security holdings of GigCapital5’s officers and directors and their respective interests as security holders in the consummation of the transactions described herein. The Registration Statement, once available, and GigCapital5 Annual Report and Form 8-K can be obtained, without charge, at the SEC’s web site (http://www.sec.gov). Investment in any securities described herein has not been approved or disapproved by the SEC or any other regulatory authority nor has any authority passed upon or endorsed the merits of the Proposed Business Combination or the accuracy or adequacy of the information contained herein. Any representation to the contrary is a criminal offense.
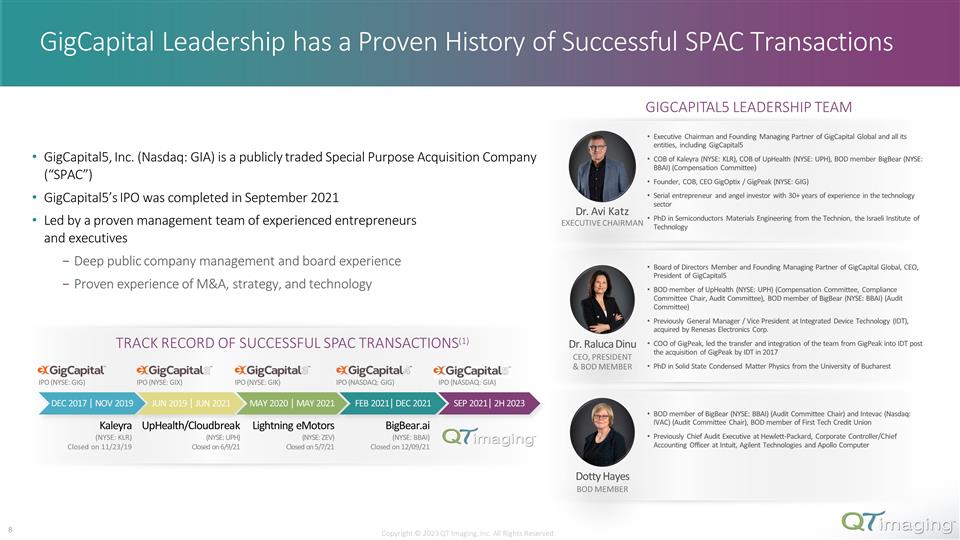
GigCapital Leadership has a Proven History of Successful SPAC Transactions GigCapital5, Inc. (Nasdaq: GIA) is a publicly traded Special Purpose Acquisition Company (“SPAC”) GigCapital5’s IPO was completed in September 2021 Led by a proven management team of experienced entrepreneurs and executives Deep public company management and board experience Proven experience of M&A, strategy, and technology Dr. Raluca Dinu CEO, PRESIDENT & BOD MEMBER Dr. Avi Katz EXECUTIVE CHAIRMAN Dotty Hayes BOD MEMBER Executive Chairman and Founding Managing Partner of GigCapital Global and all its entities, including GigCapital5 COB of Kaleyra (NYSE: KLR), COB of UpHealth (NYSE: UPH), BOD member BigBear (NYSE: BBAI) (Compensation Committee) Founder, COB, CEO GigOptix / GigPeak (NYSE: GIG) Serial entrepreneur and angel investor with 30+ years of experience in the technology sector PhD in Semiconductors Materials Engineering from the Technion, the Israeli Institute of Technology Board of Directors Member and Founding Managing Partner of GigCapital Global, CEO, President of GigCapital5 BOD member of UpHealth (NYSE: UPH) (Compensation Committee, Compliance Committee Chair, Audit Committee), BOD member of BigBear (NYSE: BBAI) (Audit Committee) Previously General Manager / Vice President at Integrated Device Technology (IDT), acquired by Renesas Electronics Corp. COO of GigPeak, led the transfer and integration of the team from GigPeak into IDT post the acquisition of GigPeak by IDT in 2017 PhD in Solid State Condensed Matter Physics from the University of Bucharest BOD member of BigBear (NYSE: BBAI) (Audit Committee Chair) and Intevac (Nasdaq: IVAC) (Audit Committee Chair), BOD member of First Tech Credit Union Previously Chief Audit Executive at Hewlett-Packard, Corporate Controller/Chief Accounting Officer at Intuit, Agilent Technologies and Apollo Computer GIGCAPITAL5 LEADERSHIP TEAM DEC 2017 | NOV 2019 IPO (NYSE: GIG) IPO (NYSE: GIX) IPO (NYSE: GIK) Kaleyra (NYSE: KLR) Closed on 11/23/19 IPO (NASDAQ: GIG) UpHealth/Cloudbreak (NYSE: UPH) Closed on 6/9/21 Lightning eMotors (NYSE: ZEV) Closed on 5/7/21 BigBear.ai (NYSE: BBAI) Closed on 12/09/21 JUN 2019 | JUN 2021 MAY 2020 | MAY 2021 FEB 2021| DEC 2021 SEP 2021| 2H 2023 IPO (NASDAQ: GIA) TRACK RECORD OF SUCCESSFUL SPAC TRANSACTIONS(1) Copyright © 2023 QT Imaging, Inc. All Rights Reserved. 2

GigCapital Recognizes QT Imaging’s Potential to Transform Medical Imaging Copyright © 2023 QT Imaging, Inc. All Rights Reserved. 2 QTI is a medical device company with imaging technology that has the potential to transform the industry QTI Scanner is the only imaging device to receive FDA clearance for use as a transmission ultrasonic imaging system of a patient’s breast QTI’s patent-protected technology provides a relatively low-cost, comprehensive, no radiation medical imaging solution yielding ~40x the resolution of MRI This sub-millimeter, high-definition, image resolution enables the identification of normal and abnormal breast structures and the accurate depiction of the precise shape and location of findings, as well as being suitable for full body imaging and other applications QTI is led by CEO John Klock, MD, who is recognized globally as a successful co-founder of multiple companies, including one that successfully commercialized five FDA-approved drugs Why GigCapital selected QT Imaging (“QTI”):
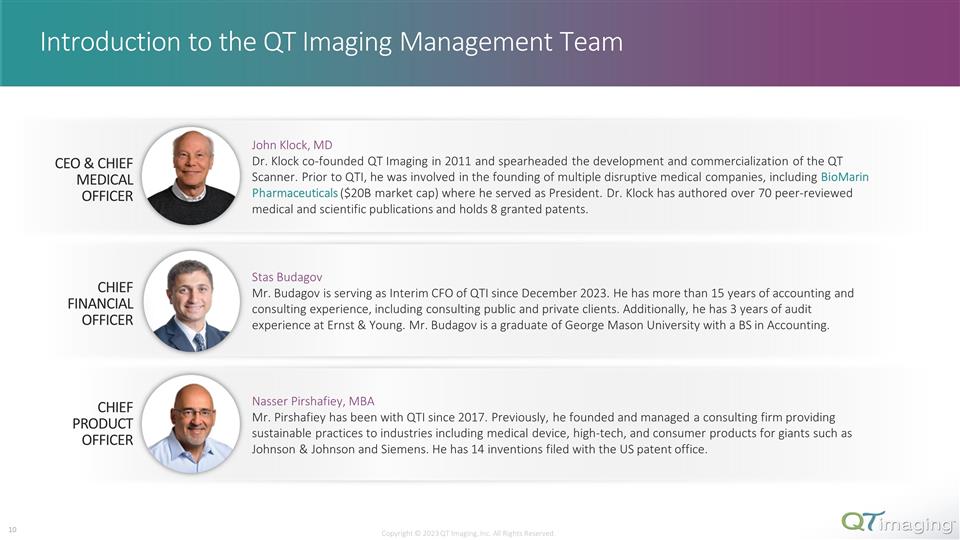
Introduction to the QT Imaging Management Team CEO & CHIEF MEDICAL OFFICER CHIEF PRODUCT OFFICER John Klock, MD Dr. Klock co-founded QT Imaging in 2011 and spearheaded the development and commercialization of the QT Scanner. Prior to QTI, he was involved in the founding of multiple disruptive medical companies, including BioMarin Pharmaceuticals ($20B market cap) where he served as President. Dr. Klock has authored over 70 peer- reviewed medical and scientific publications and holds 8 granted patents. Stas Budagov Mr. Budagov is serving as Interim CFO of QTI since December 2023. He has more than 15 years of accounting and consulting experience, including consulting public and private clients. Additionally, he has 3 years of audit experience at Ernst & Young. Mr. Budagov is a graduate of George Mason University with a BS in Accounting. Nasser Pirshafiey, MBA Mr. Pirshafiey has been with QTI since 2017. Previously, he founded and managed a consulting firm providing sustainable practices to industries including medical device, high-tech, and consumer products for giants such as Johnson & Johnson and Siemens. He has 14 inventions filed with the US patent office. CHIEF FINANCIAL OFFICER Copyright © 2023 QT Imaging, Inc. All Rights Reserved. 3
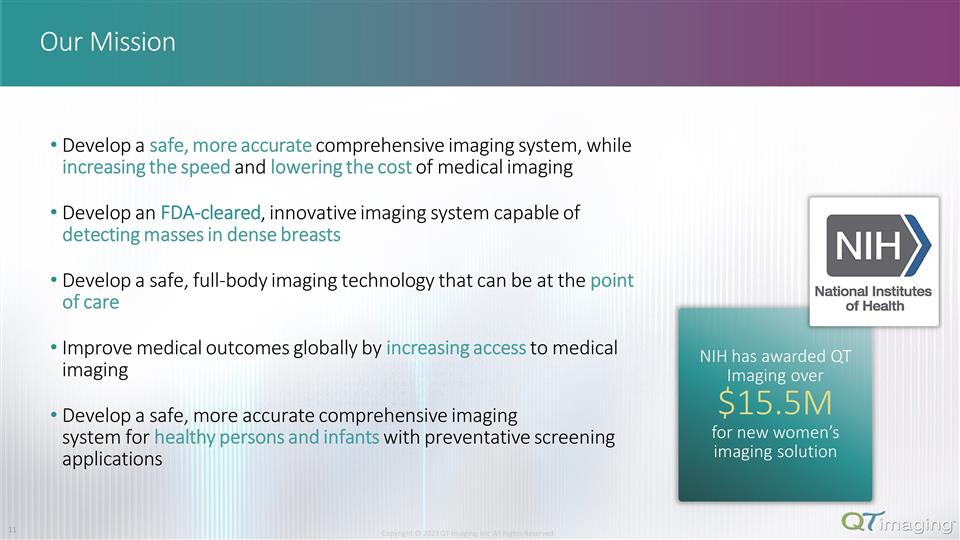
Our Mission Develop a safe, more accurate comprehensive imaging system, while increasing the speed and lowering the cost of medical imaging Develop an FDA-cleared, innovative imaging system capable of detecting masses in dense breasts Develop a safe, full-body imaging technology that can be at the point of care Improve medical outcomes globally by increasing access to medical imaging Develop a safe, more accurate comprehensive imaging system for healthy persons and infants with preventative screening applications NIH has awarded QT Imaging over $15.5M for new women’s imaging solution Copyright © 2023 QT Imaging, Inc. All Rights Reserved. 4
Executive Summary Low-cost, comprehensive, no radiation medical imaging solution yielding sub-millimeter, high-definition, image resolution: application in areas such as breast • infant body • orthopedics Commercial stage, FDA-cleared(1) breast scanner for dense breast imaging, with better sensitivity and specificity than mammography and potential for: Applicability to determine breast density and measure mass size and growth Improved compliance with screening guidelines Expanded FDA clearances to increase access to medical imaging in multiple applications, including preventative screening Breakthrough Device Designation awarded by the FDA provides fast track to unique CPT codes and future clearances Patent-protected technology: 12 granted US/Europe • 1 pending Software platform protected by trade secrets Sales Agent Agreement signed with NXC Imaging (A Subsidiary of Canon Medical Systems) Go-to-market strategy: US: Combination of direct sales force and distributor network OUS / Global: Partnerships with strategics & distributors in key regions Asia • Europe • Middle East • North Africa Developed roadmap for additional FDA clearances, product development, clinical adoption, and commercialization Experienced management team supported by successful SPAC management team Copyright © 2023 QT Imaging, Inc. All Rights Reserved. FDA Labels: K162372, K181785, K190626, KD22093, Q181785 5
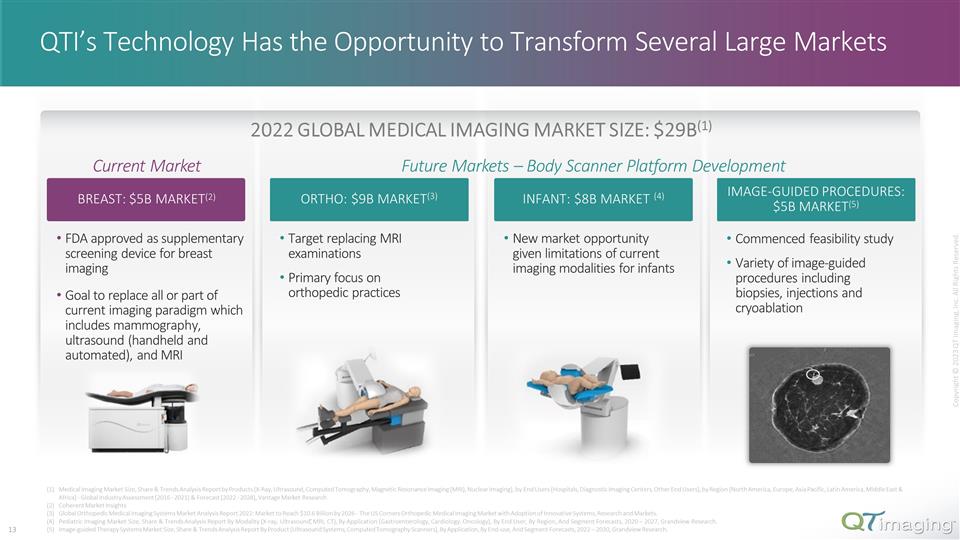
. New market opportunity given limitations of current imaging modalities for infants Commenced feasibility study Variety of image-guided procedures including biopsies, injections and cryoablation QTI’s Technology Has the Opportunity to Transform Several Large Markets Current Market FDA approved as supplementary screening device for breast imaging Goal to replace all or part of current imaging paradigm which includes mammography, ultrasound (handheld and automated), and MRI INFANT: $8B MARKET (4) IMAGE-GUIDED PROCEDURES: $5B MARKET(5) BREAST: $5B MARKET(2) ORTHO: $9B MARKET(3) Target replacing MRI examinations Primary focus on orthopedic practices Medical Imaging Market Size, Share & Trends Analysis Report by Products (X-Ray, Ultrasound, Computed Tomography, Magnetic Resonance Imaging (MRI), Nuclear Imaging), by End Users (Hospitals, Diagnostic Imaging Centers, Other End Users), by Region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa) - Global Industry Assessment (2016 - 2021) & Forecast (2022 - 2028), Vantage Market Research Coherent Market Insights Global Orthopedic Medical Imaging Systems Market Analysis Report 2022: Market to Reach $10.6 Billion by 2026 - The US Corners Orthopedic Medical Imaging Market with Adoption of Innovative Systems, Research and Markets. Pediatric Imaging Market Size, Share & Trends Analysis Report By Modality (X-ray, Ultrasound, MRI, CT), By Application (Gastroenterology, Cardiology, Oncology), By End User, By Region, And Segment Forecasts, 2020 – 2027, Grandview Research. Image-guided Therapy Systems Market Size, Share & Trends Analysis Report By Product (Ultrasound Systems, Computed Tomography Scanners), By Application, By End-use, And Segment Forecasts, 2022 – 2030, Grandview Research. 2022 GLOBAL MEDICAL IMAGING MARKET SIZE: $29B(1) Copyright © 2023 QT Imaging, Inc. All Rights Reserved. Future Markets – Body Scanner Platform Development 6
Copyright © 2023 QT Imaging, Inc. All Rights Reserved. Sales Agent Agreement signed with NXC Imaging marks a major milestone for QT Imaging Accessing NXC Imaging's distribution channel in the US and the US territories, this agreement provides potential to accelerate the commercial roll-out of QTI’s imaging system NXC Imaging will also provide a mature service organization to support the QT Imaging’s installed base Agreement Signed with NXC Imaging (A Subsidiary of Canon Medical Systems)
Investment Highlights Cutting-edge imaging technology with multiple potential applications creates a tremendous opportunity to transform the imaging market Experienced and Committed Executive Suite Industry-Transforming Imaging Technology Platform Recognized by Industry Incumbents Recent Changes to FDA Rules and USPSTF Guidance on Breast Screening Provide Meaningful Tailwinds and Momentum Differentiated Solution in Large and Important, $5B(1) Breast Screening Market Potential to Significantly Expand TAM Through Adjacent Market Applications NXC Imaging Agreement to Drive Accelerated Commercial Roll-out Coherent Market Insights

TECHNOLOGY OVERVIEW 8

Current Ultrasound Technologies Have Major Deficiencies Clinically useful sensitivity and specificity Presence of comparative clinical trials Proven success in head-to-head trials against mammography for primary screening Ability for doubling times – can identify slow growing cancers and help prevent cancer deaths Enhanced volume measurements – can follow cancer treatments and provide breast density measurements Patented technology opens the door for potential future growth in orthopedic and pediatric imaging Critical Modality Advantages of QTI(2) Reflection and compounding artifacts No valid true “transmission” mode – use “shear wave” (low resolution) data Data yielded is compounded 2D – not true “3D” “Speed” photos provide compromised resolution Low contrast-to-noise ratios Specificity for masses is poor Unable to view calcifications – misses 20% of cancers(1) No “functional” imaging features (doubling time, tissue identification and specific tissue volume segmentations) Poor reproducibility of measurement and volume data Shortfalls of Current, Rival Systems 9 Based on opinion of QT Imaging. See Form S4/A for further details/explanation Based on opinion of QT Imaging. QTI believes necessary data has been obtained through 18 separate clinical trials
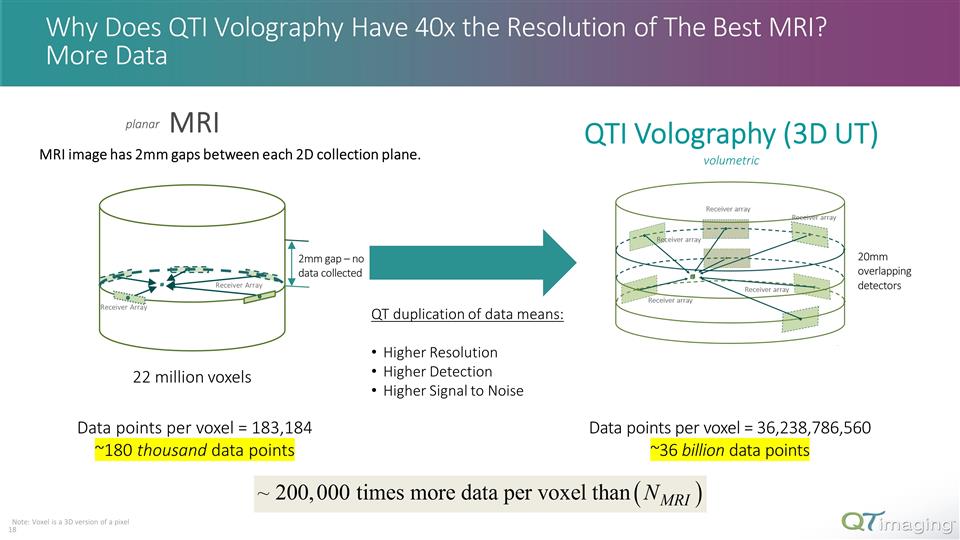
Why Does QTI Volography Have 40x the Resolution of The Best MRI? More Data MRI QTI Volography (3D UT) volumetric planar QT duplication of data means: Higher Resolution Higher Detection Higher Signal to Noise Note: Voxel is a 3D version of a pixel 10 MRI image has 2mm gaps between each 2D collection plane. Data points per voxel = 36,238,786,560 ~36 billion data points 22 million voxels Data points per voxel = 183,184 ~180 thousand data points Receiver Array Receiver Array 2mm gap – no data collected 20mm overlapping detectors
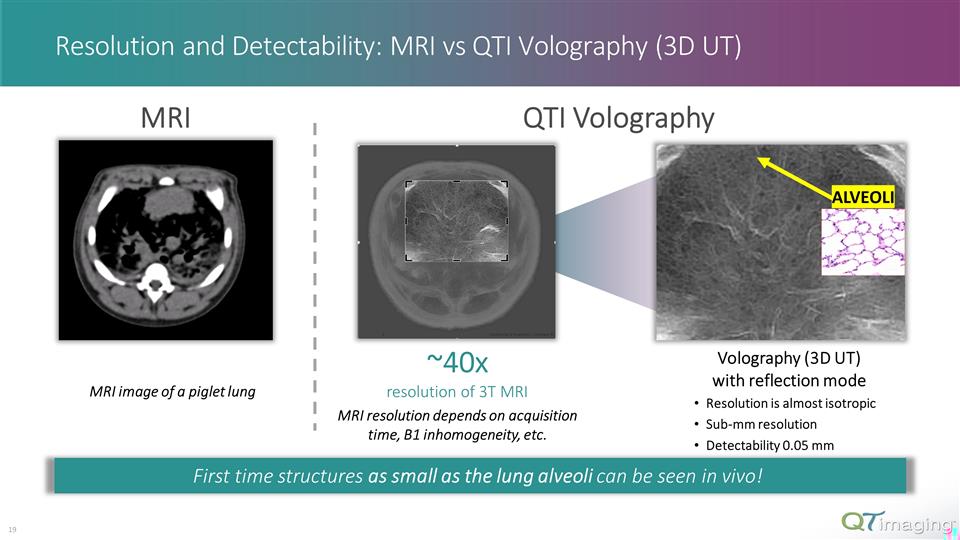
Volography (3D UT) with reflection mode Resolution is almost isotropic Sub-mm resolution Detectability 0.05 mm ~40x resolution of 3T MRI MRI resolution depends on acquisition time, B1 inhomogeneity, etc. Resolution and Detectability: MRI vs QTI Volography (3D UT) First time structures as small as the lung alveoli can be seen in vivo! ALVEOLI 11 MRI image of a piglet lung MRI QTI Volography

BREAST HEALTH 15

Copyright © 2023 QT Imaging, Inc. All Rights Reserved. QT Imaging’s FDA-cleared Solution for Dense Breasts 50% of women between the ages of 40-74 in the US have dense breasts(1) MANY WOMEN HAVE DENSE BREASTS, WHICH MAMMOGRAMS ARE INEFFICIENT IN SCREENING FOR CANCER Dense BreastOther 50% 50% Mammography Misses 35.6–52.2% Of Breast Cancers In Dense Breast Tissue(4) Breast Density on a Mammogram, Susan G. Komen QTI Study | Dense Breast Mass Detection “Mammograms Must Include Breast Density Information, New FDA Rule Says”. Wall Street Journal The Role of Ultrasound in Screening Dense Breasts. NCBI. THE FDA HAS RECOGNIZED THE IMPORTANCE OF BREAST DENSITY IN BREAST CANCER SCREENING “the new rule advises physicians and patients to consider breast density alongside other cancer risk factors when deciding whether additional screening is necessary” – Hilary Marston, Chief Medical Officer FDA In ~84% of cases observed in a recent mini-study, QT Scanner identified abnormalities in dense breasts that were not identified by x-ray mammograms X-Ray Mammogram QT Scan (2) 16 (3)
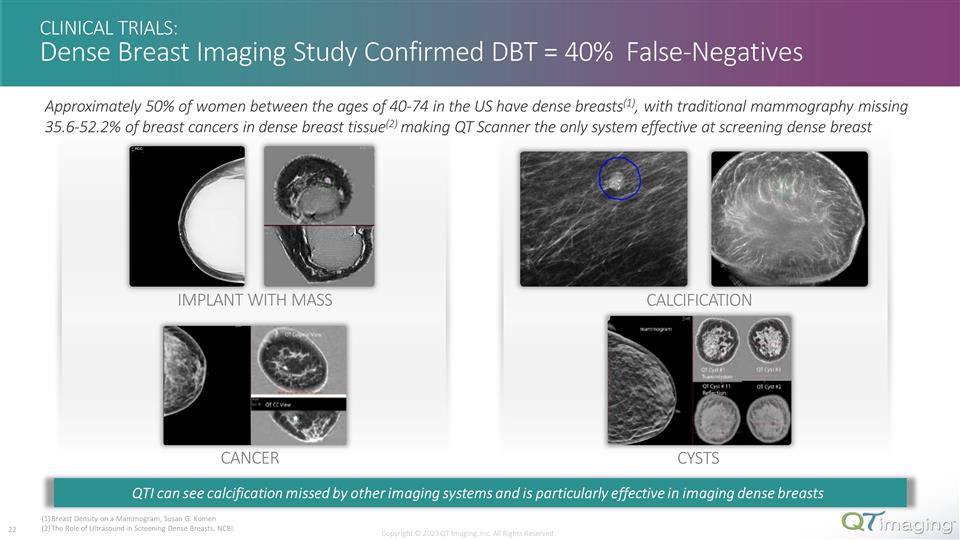
CYSTS IMPLANT WITH MASS CANCER CLINICAL TRIALS: Dense Breast Imaging Study Confirmed DBT = 40% False-Negatives Copyright © 2023 QT Imaging, Inc. All Rights Reserved. Approximately 50% of women between the ages of 40-74 in the US have dense breasts(1), with traditional mammography missing 35.6-52.2% of breast cancers in dense breast tissue(2) making QT Scanner the only system effective at screening dense breast Breast Density on a Mammogram, Susan G. Komen The Role of Ultrasound in Screening Dense Breasts. NCBI. QTI can see calcification missed by other imaging systems and is particularly effective in imaging dense breasts CALCIFICATION 12
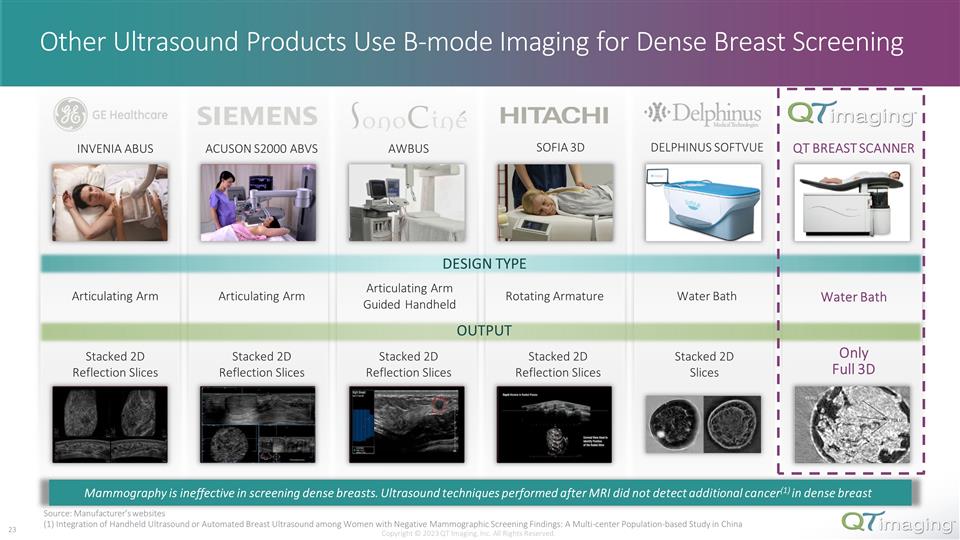
INVENIA ABUS ACUSON S2000 ABVS AWBUS SOFIA 3D DELPHINUS SOFTVUE QT BREAST SCANNER DESIGN TYPE Articulating Arm Articulating Arm Articulating Arm Guided Handheld Rotating Armature Water Bath Water Bath OUTPUT Stacked 2D Reflection Slices Stacked 2D Reflection Slices Stacked 2D Reflection Slices Stacked 2D Reflection Slices Stacked 2D Slices Only Full 3D Other Ultrasound Products Use B-mode Imaging for Dense Breast Screening Source: Manufacturer’s websites (1) Integration of Handheld Ultrasound or Automated Breast Ultrasound among Women with Negative Mammographic Screening Findings: A Multi-center Population-based Study in China Copyright © 2023 QT Imaging, Inc. All Rights Reserved. Mammography is ineffective in screening dense breasts. Ultrasound techniques performed after MRI did not detect additional cancer(1) in dense breast 22
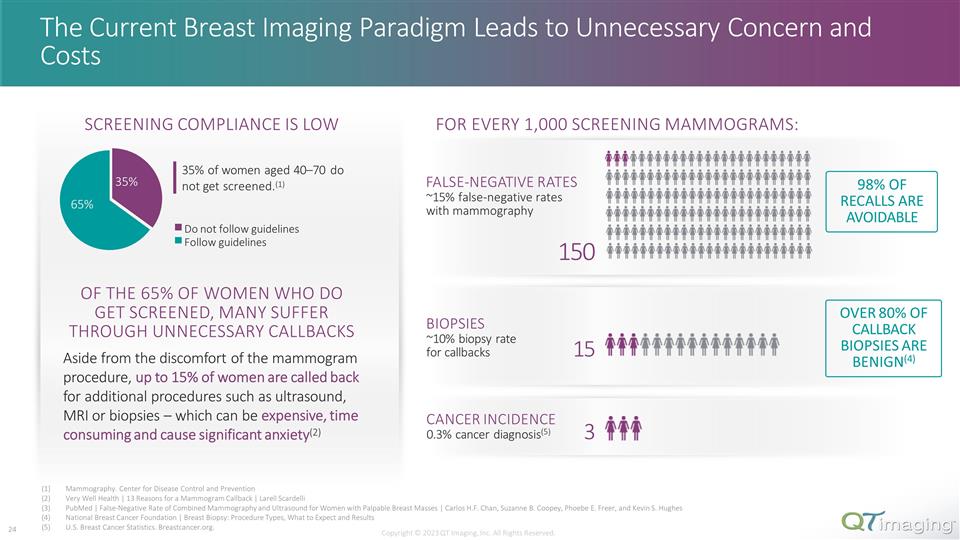
35% 65% Do not follow guidelines Follow guidelines OVER 80% OF CALLBACK BIOPSIES ARE BENIGN(4) 98% OF RECALLS ARE AVOIDABLE Mammography. Center for Disease Control and Prevention Very Well Health | 13 Reasons for a Mammogram Callback | Larell Scardelli PubMed | False-Negative Rate of Combined Mammography and Ultrasound for Women with Palpable Breast Masses | Carlos H.F. Chan, Suzanne B. Coopey, Phoebe E. Freer, and Kevin S. Hughes National Breast Cancer Foundation | Breast Biopsy: Procedure Types, What to Expect and Results U.S. Breast Cancer Statistics. Breastcancer.org. The Current Breast Imaging Paradigm Leads to Unnecessary Concern and Costs FALSE-NEGATIVE RATES ~15% false-negative rates with mammography BIOPSIES ~10% biopsy rate for callbacks CANCER INCIDENCE 0.3% cancer diagnosis(5) 150 15 3 FOR EVERY 1,000 SCREENING MAMMOGRAMS: 35% of women aged 40–70 do not get screened.(1) SCREENING COMPLIANCE IS LOW Copyright © 2023 QT Imaging, Inc. All Rights Reserved. OF THE 65% OF WOMEN WHO DO GET SCREENED, MANY SUFFER THROUGH UNNECESSARY CALLBACKS Aside from the discomfort of the mammogram procedure, up to 15% of women are called back for additional procedures such as ultrasound, MRI or biopsies – which can be expensive, time consuming and cause significant anxiety(2) 17
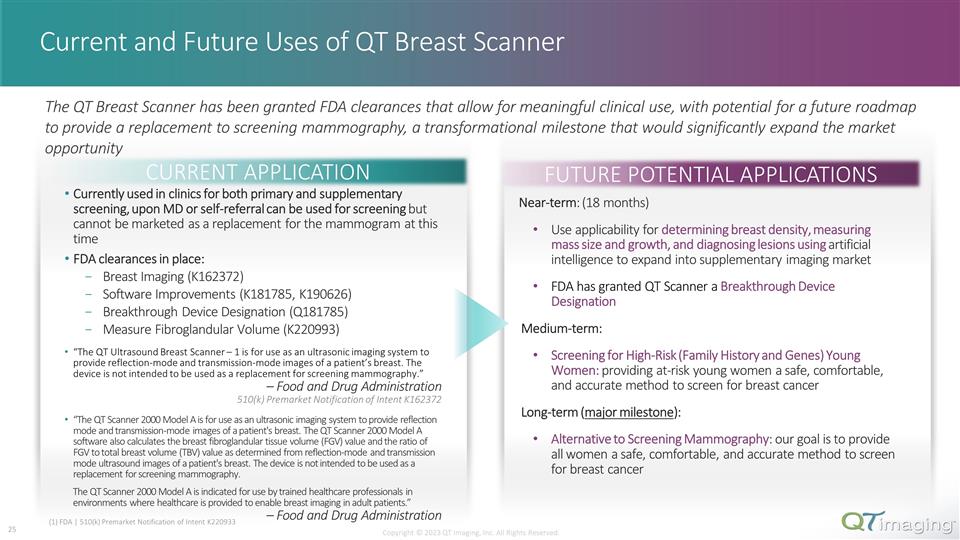
Current and Future Uses of QT Breast Scanner Copyright © 2023 QT Imaging, Inc. All Rights Reserved. CURRENT APPLICATION FUTURE POTENTIAL APPLICATIONS Currently used in clinics for both primary and supplementary screening, upon MD or self-referral can be used for screening but cannot be marketed as a replacement for the mammogram at this time FDA clearances in place: Breast Imaging (K162372) Software Improvements (K181785, K190626) Breakthrough Device Designation (Q181785) Measure Fibroglandular Volume (K220993) “The QT Ultrasound Breast Scanner – 1 is for use as an ultrasonic imaging system to provide reflection-mode and transmission-mode images of a patient’s breast. The device is not intended to be used as a replacement for screening mammography.” – Food and Drug Administration 510(k) Premarket Notification of Intent K162372 “The QT Scanner 2000 Model A is for use as an ultrasonic imaging system to provide reflection mode and transmission-mode images of a patient's breast. The QT Scanner 2000 Model A software also calculates the breast fibroglandular tissue volume (FGV) value and the ratio of FGV to total breast volume (TBV) value as determined from reflection-mode and transmission mode ultrasound images of a patient's breast. The device is not intended to be used as a replacement for screening mammography. The QT Scanner 2000 Model A is indicated for use by trained healthcare professionals in environments where healthcare is provided to enable breast imaging in adult patients.” – Food and Drug Administration Near-term: (18 months) Use applicability for determining breast density, measuring mass size and growth, and diagnosing lesions using artificial intelligence to expand into supplementary imaging market FDA has granted QT Scanner a Breakthrough Device Designation Medium-term: Screening for High-Risk (Family History and Genes) Young Women: providing at-risk young women a safe, comfortable, and accurate method to screen for breast cancer Long-term (major milestone): Alternative to Screening Mammography: our goal is to provide all women a safe, comfortable, and accurate method to screen for breast cancer The QT Breast Scanner has been granted FDA clearances that allow for meaningful clinical use, with potential for a future roadmap to provide a replacement to screening mammography, a transformational milestone that would significantly expand the market opportunity (1) FDA | 510(k) Premarket Notification of Intent K220933 18
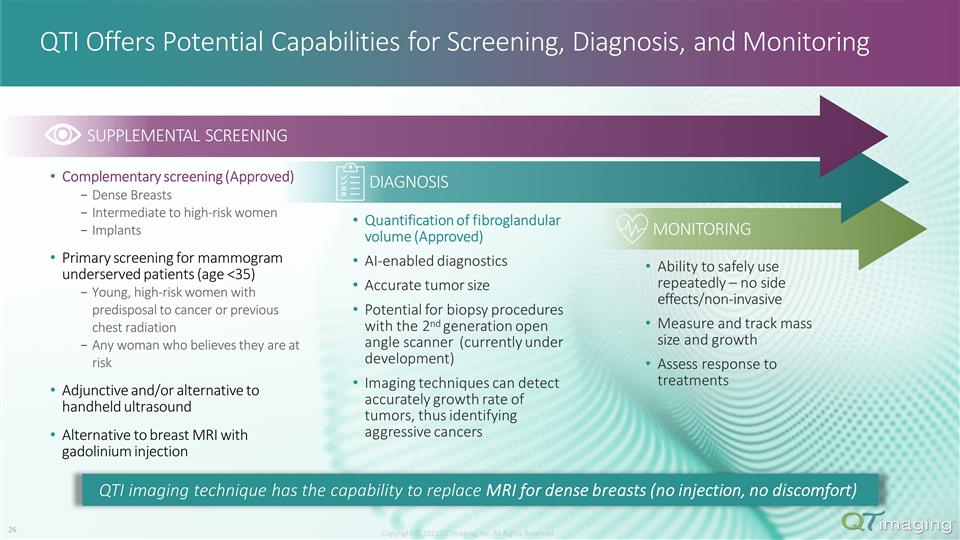
QTI Offers Potential Capabilities for Screening, Diagnosis, and Monitoring Complementary screening (Approved) Dense Breasts Intermediate to high-risk women Implants Primary screening for mammogram underserved patients (age <35) Young, high-risk women with predisposal to cancer or previous chest radiation Any woman who believes they are at risk Adjunctive and/or alternative to handheld ultrasound Alternative to breast MRI with gadolinium injection DIAGNOSIS SUPPLEMENTAL SCREENING MONITORING Quantification of fibroglandular volume (Approved) AI-enabled diagnostics Accurate tumor size Potential for biopsy procedures with the 2nd generation open angle scanner (currently under development) Imaging techniques can detect accurately growth rate of tumors, thus identifying aggressive cancers Ability to safely use repeatedly – no side effects/non-invasive Measure and track mass size and growth Assess response to treatments Copyright © 2023 QT Imaging, Inc. All Rights Reserved. QTI imaging technique has the capability to replace MRI for dense breasts (no injection, no discomfort) 19
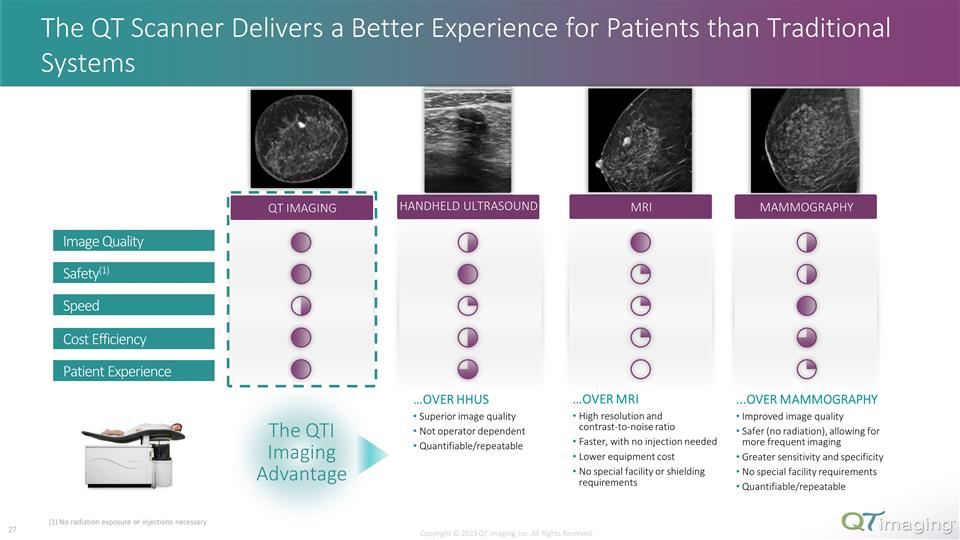
The QT Scanner Delivers a Better Experience for Patients than Traditional Systems QT IMAGING MRI HANDHELD ULTRASOUND MAMMOGRAPHY Copyright © 2023 QT Imaging, Inc. All Rights Reserved. Image Quality Safety(1) Speed Cost Efficiency Patient Experience 21 The QTI Imaging Advantage ....OVER MAMMOGRAPHY Improved image quality Safer (no radiation), allowing for more frequent imaging Greater sensitivity and specificity No special facility requirements Quantifiable/repeatable …OVER HHUS Superior image quality Not operator dependent Quantifiable/repeatable …OVER MRI High resolution and contrast-to-noise ratio Faster, with no injection needed Lower equipment cost No special facility or shielding requirements (1) No radiation exposure or injections necessary
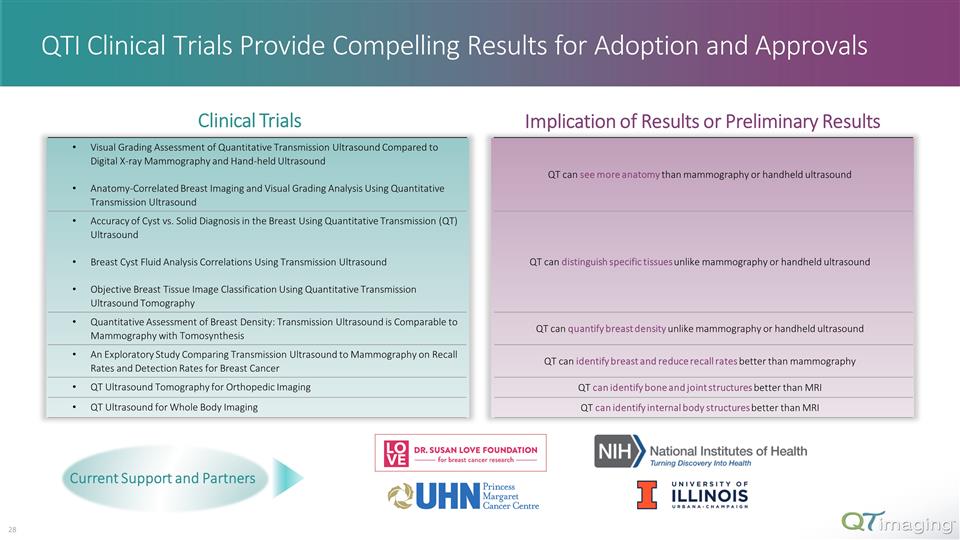
Visual Grading Assessment of Quantitative Transmission Ultrasound Compared to Digital X-ray Mammography and Hand-held Ultrasound Anatomy-Correlated Breast Imaging and Visual Grading Analysis Using Quantitative Transmission Ultrasound QT can see more anatomy than mammography or handheld ultrasound Accuracy of Cyst vs. Solid Diagnosis in the Breast Using Quantitative Transmission (QT) Ultrasound Breast Cyst Fluid Analysis Correlations Using Transmission Ultrasound Objective Breast Tissue Image Classification Using Quantitative Transmission Ultrasound Tomography QT can distinguish specific tissues unlike mammography or handheld ultrasound Quantitative Assessment of Breast Density: Transmission Ultrasound is Comparable to Mammography with Tomosynthesis QT can quantify breast density unlike mammography or handheld ultrasound An Exploratory Study Comparing Transmission Ultrasound to Mammography on Recall Rates and Detection Rates for Breast Cancer QT can identify breast and reduce recall rates better than mammography QT Ultrasound Tomography for Orthopedic Imaging QT can identify bone and joint structures better than MRI QT Ultrasound for Whole Body Imaging QT can identify internal body structures better than MRI QTI Clinical Trials Provide Compelling Results for Adoption and Approvals Clinical Trials Implication of Results or Preliminary Results Current Support and Partners
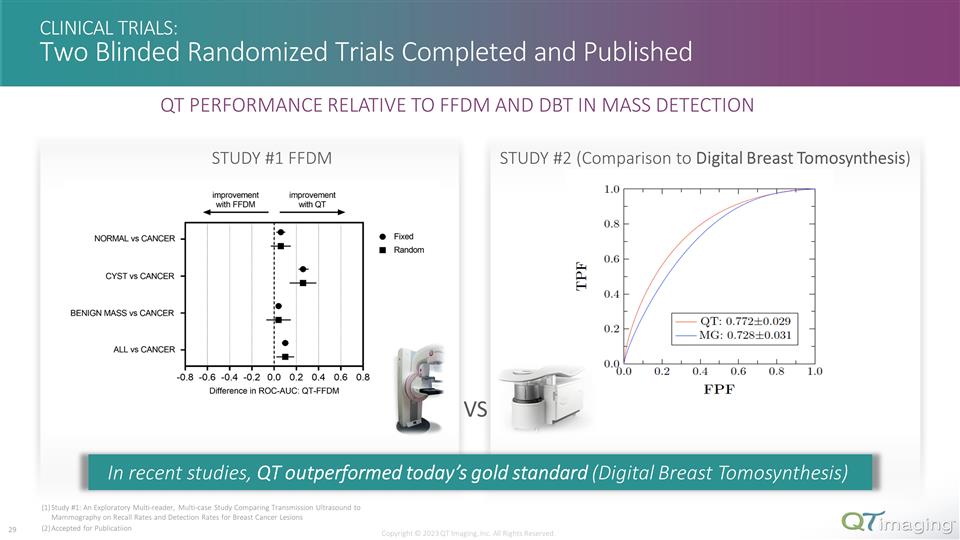
CLINICAL TRIALS: Two Blinded Randomized Trials Completed and Published VS STUDY #1 FFDM STUDY #2 (Comparison to Digital Breast Tomosynthesis) Copyright © 2023 QT Imaging, Inc. All Rights Reserved. Study #1: An Exploratory Multi-reader, Multi-case Study Comparing Transmission Ultrasound to Mammography on Recall Rates and Detection Rates for Breast Cancer Lesions Accepted for Publicatiion In recent studies, QT outperformed today’s gold standard (Digital Breast Tomosynthesis) 13 QT PERFORMANCE RELATIVE TO FFDM AND DBT IN MASS DETECTION

CLINICAL ADOPTION 23

Key Milestones Have Been Achieved, with Additional Catalysts to Drive Commercial Adoption and Increased Market Share Key Milestones Achieved for Commercial Adoption Four placements in North America Three placements internationally Generate and publish clinical data Develop market advocates Signed Sales Agent Agreement with NXC Imaging (A Subsidiary of Canon Medical Systems) for worldwide sales and service rollout Catalysts for Further Commercial Adoption Screening adjunct clearance for high-risk young women Primary screening clearance for all women subject to FDA approval Product enhancements while further developing sales and marketing team FDA Clearance for Primary Screening MAJOR MILESTONE KEY MILESTONES BREAST SCANNER 18 MONTHS Copyright © 2023 QT Imaging, Inc. All Rights Reserved. Millions of young, at-risk women can benefit from QTI’s potential FDA clearance for primary screening 24
Traditional Upfront Purchase MSaaS (Medical Scan as a Service)/Per Click Model Turnkey Model (includes scan interpretation) * all require annual maintenance and custom disposables Pricing Structures Allow Providers Flexibility in Using the QT Scanner INITIAL TARGET MARKETS Community Cancer Centers Academic Medical Centers Private Practices Independent Breast Imaging Centers PRICING MODEL* Copyright © 2023 QT Imaging, Inc. All Rights Reserved. 25

Reimbursement Will Be Driven by the Value and Savings Provided to Patients Copyright © 2023 QT Imaging, Inc. All Rights Reserved. CURRENT Existing CPT codes, non-specific to QTI technology: Unilateral or Bilateral breast ultrasound (76641 or 76642) 3D rendering (76377) Other ultrasound procedures (76999) FUTURE CPT code specific to QT Scanner® Higher reimbursements capture full value of unique advantages that QT Scans offer Process to QTI-specific code facilitated by breakthrough designation Reimbursement agreements with specific insurance companies and programs Integrated health systems focused on minimizing overall cost of care Programs serving higher risk groups 26

OPEN ANGLE SCANNER 27
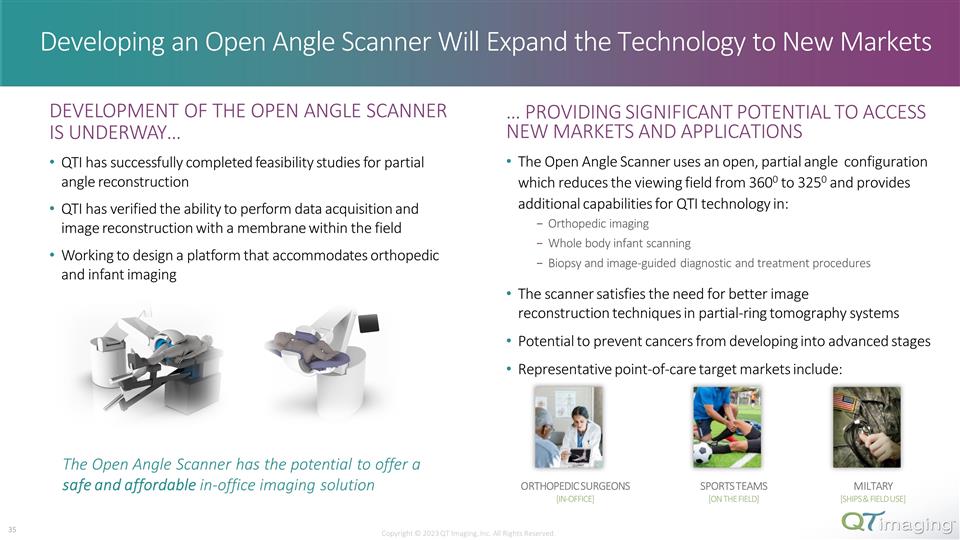
Developing an Open Angle Scanner Will Expand the Technology to New Markets … PROVIDING SIGNIFICANT POTENTIAL TO ACCESS NEW MARKETS AND APPLICATIONS The Open Angle Scanner uses an open, partial angle configuration which reduces the viewing field from 3600 to 3250 and provides additional capabilities for QTI technology in: Orthopedic imaging Whole body infant scanning Biopsy and image-guided diagnostic and treatment procedures The scanner satisfies the need for better image reconstruction techniques in partial-ring tomography systems Potential to prevent cancers from developing into advanced stages Representative point-of-care target markets include: Copyright © 2023 QT Imaging, Inc. All Rights Reserved. DEVELOPMENT OF THE OPEN ANGLE SCANNER IS UNDERWAY… QTI has successfully completed feasibility studies for partial angle reconstruction QTI has verified the ability to perform data acquisition and image reconstruction with a membrane within the field Working to design a platform that accommodates orthopedic and infant imaging The Open Angle Scanner has the potential to offer a safe and affordable in-office imaging solution ORTHOPEDIC SURGEONS [IN-OFFICE] SPORTS TEAMS [ON THE FIELD] MILTARY [SHIPS & FIELD USE] 28
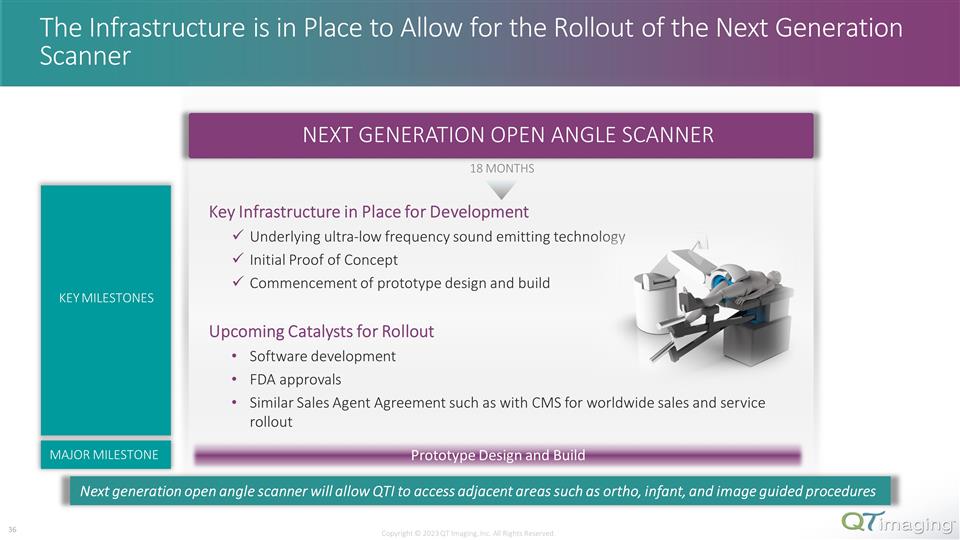
The Infrastructure is in Place to Allow for the Rollout of the Next Generation Scanner Prototype Design and Build MAJOR MILESTONE NEXT GENERATION OPEN ANGLE SCANNER 18 MONTHS Copyright © 2023 QT Imaging, Inc. All Rights Reserved. 24 Key Infrastructure in Place for Development Underlying ultra-low frequency sound emitting technology Initial Proof of Concept Commencement of prototype design and build KEY MILESTONES Next generation open angle scanner will allow QTI to access adjacent areas such as ortho, infant, and image guided procedures Upcoming Catalysts for Rollout Software development FDA approvals Similar Sales Agent Agreement such as with CMS for worldwide sales and service rollout
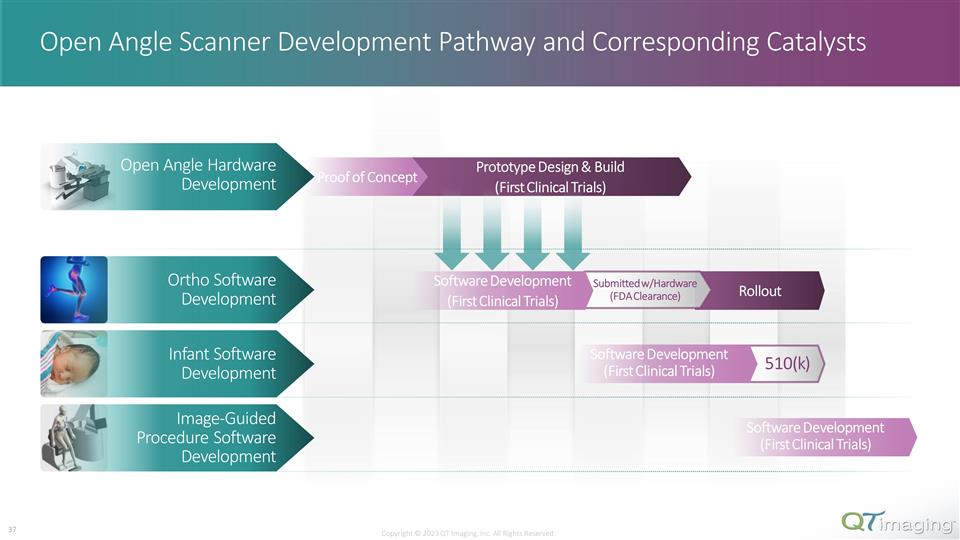
Open Angle Scanner Development Pathway and Corresponding Catalysts 510(k) Ortho Software Development Infant Software Development Image-Guided Procedure Software Development Prototype Design & Build (First Clinical Trials) Proof of Concept Open Angle Hardware Development Rollout Submitted w/Hardware (FDA Clearance) 510(k) Software Development (First Clinical Trials) Software Development (First Clinical Trials) Software Development (First Clinical Trials) Copyright © 2023 QT Imaging, Inc. All Rights Reserved. 29

Copyright © 2024 QT Imaging, Inc. All Rights Reserved. Risk Factors All references to “QT Imaging,” “Company,” “we,” “us” or “our” refer to the business of QT Imaging, Inc. The risks presented below are certain of the general risks of the Company, GigCapital5, Inc (“SPAC”), and the proposed transaction between the Company and the SPAC (the “Proposed Business Combination”). You should carefully consider these risks and uncertainties, together with the information in this Presentation and the Company’s financial statements and related notes when filed with the U.S. Securities and Exchange Commission, and you should carry out your own diligence and consult with your own financial and legal advisors concerning the risks before making any investment decisions. Legal, Regulatory and Compliance Risks QT Imaging’s medical device scanners, as well as our business operations and activities, are subject to extensive regulation and compliance obligations, as well as rigorous enforcement, including by the U.S. FDA and numerous other federal, state, and non-U.S. governmental authorities. We cannot guarantee that we will be able to obtain or maintain marketing clearance for our new products or enhancements or modifications to existing products. The failure to maintain approvals or obtain approval or clearance could have a material adverse effect on our business. The use, misuse or off-label use of our products may result in injuries that lead to product liability suits, which could be costly to our business. Healthcare reform measures could hinder or prevent the commercial success of our business. If clinical studies for future indications do not produce results necessary to support regulatory clearance or approval in the U.S. or elsewhere, we will be unable to commercialize our products for these indications. Failure to obtain regulatory approvals in foreign jurisdictions will prevent us from marketing our products internationally. Unauthorized third parties may seek to access our devices or our products and services, or related devices, products and services and modify or use them in a way inconsistent with our FDA clearances and approvals, which may create risks to users. Our products may be subject to recalls after receiving FDA or foreign approval or clearance which could divert managerial and financial resources, harm or reputation and adversely affect our business. If we fail to comply with U.S. federal and state fraud and abuse and other healthcare laws and regulations, including those relating to kickbacks and false claims, we could face substantial penalties and our business operations and financial condition could be harmed.

Copyright © 2024 QT Imaging, Inc. All Rights Reserved. Risk Factors – Cont. Development Stage Technology Risks The market for the Company’s medical scanner is subject to technological change. The success of the Company depends on the timely perception of new trends, developments and customer needs, constant further development of engineering expertise and ensuring that products and services keep pace with technological developments. Our competitors may launch new products and services earlier or at more competitive prices or secure exclusive rights to new technologies. If our competitors gain advantages in alternative technologies, this could affect the competitive position of the Company. If these circumstances materialize, it may have a significant adverse effect on the company’s business, prospects, financial results, or results of operations. Defects or failures associated with our products could lead to recalls, safety alerts or litigation, as well as significant costs and negative publicity. Adoption of our product depends upon appropriate healthcare provider training. Inadequate training may lead to negative patient outcomes, effect adoption of our products and adversely affect our business. Risks Related to Our Business We have a history of generating net losses, and if we are unable to achieve adequate revenue growth while our expenses increase, we may not achieve or maintain profitability in the future. If we fail to manage our growth effectively or to sustain our revenue growth, we may be unable to execute our business plan, maintain customer satisfaction or adequately address competitive challenges. Members of the Company’s management have limited experience in operating a public company. The requirements of being a public company may strain our resources and divert management’s attention, and the increases in legal, accounting and compliance expenses that will result from being a public company may be greater than we anticipate. If we do not attract new customers and increase our customers’ use of our products and services, our business will suffer. Our ability to recruit, retain, and develop qualified personnel is critical to our success and growth. If our technical and maintenance support services are not satisfactory to our customers, they may not buy future products, which could materially and adversely affect our future results of operations and financial condition. Any future litigation against us could be costly and time-consuming to defend. Our pricing decisions and pricing models may adversely affect our ability to attract new customers and retain existing customers. We may require additional capital, which additional financing may result in restrictions on our operations or substantial dilution to our stockholders, to support the growth of our business, and this capital might not be available on acceptable terms, if at all. The estimates of market opportunity and forecasts of market growth included in this presentation may prove to be inaccurate, and even if the market in which we compete achieves the forecasted growth, our business could fail to grow at similar rates, if at all.
Copyright © 2024 QT Imaging, Inc. All Rights Reserved. Risk Factors – Cont. Intellectual Property Risks Although the Company has been granted several patents, no assurances can be given that the scope of any patent protection will exclude competitors or provide competitive advantages to the Company, that anticipated and/or desired additional patents will be awarded, that any of the Company’s patents will be held valid if challenged or that others will not claim rights in them, or that the Company’s existing patents will not be designed around or improved upon by others. A loss of any of the Company’s key intellectual property rights would be detrimental to the Company’s prospects. If we fail to adequately protect our intellectual property rights, our competitive position could be impaired, and we may lose valuable assets, generate reduced revenue and become subject to ligation to protect our rights. We may be subject to claims by third parties of intellectual property infringement. We use open-source software in our devices, which could negatively affect our ability to sell our services or subject us to litigation or other actions. Third- party claims that we are infringing intellectual property, whether successful or not, could subject us to costly and time-consuming litigation or expensive licenses, and or business could be adversely affected. Our intellectual property applications, including patent applications, may not be approved of granted or may take longer than expected to be approved, which may have a material adverse effect on our ability to prevent others from commercial exploiting products similar to ours. In addition to patented, technology, we rely on trade secrets, designs, experiences, workflows, data processes, software and know-how. Reimbursement of Services Most of QT Imaging’s customer pool, and the health care providers to whom their customers supply medical services, rely on third-party payers, including government programs and private health insurance plans, to reimburse some or all of the cost of the procedures for which QT Imaging manufactures or produces medical scanners. If third-party payer coverage and adequate reimbursement cannot be obtained, sales of the Company’s products and the ability to sell our products profitably may decline significantly. Changes to reimbursement rates and measures to reduce healthcare costs may adversely impact our business. Financing Risk The Company will need to raise additional capital to fund its current business plan. It is probable that the Company’s plans will change; such changes may require more capital than currently planned. Financing may not be available or may only be available on unfavorable terms. If the Company cannot raise adequate funds to satisfy its capital requirements, the Company may need to significantly alter or limit its operations.

Copyright © 2024 QT Imaging, Inc. All Rights Reserved. Risk Factors – Cont. General Business Risks Downturns or volatility in general economic conditions could have a material adverse effect on the Company’s business, financial condition, results of operations and liquidity. The Company's competitive position could be adversely affected if it is unable to meet customers’ quality requirements. If the Company is unable to expand or further diversify its customer base, its business, financial condition, and results of operations could suffer. If the Company’s products do not conform to, or are not compatible with, existing or emerging industry standards, demand for its products may decrease, which in turn would harm the Company’s business and operating results. The Company is subject to risks and uncertainties associated with international operations, which may harm its business. The Company's company culture has contributed to its success and if the Company cannot maintain this culture as it grows, its business could be harmed. The Company may not be able to effectively manage its growth and may need to incur significant expenditures to address the additional operational and control requirements of its growth, either of which could harm the Company’s business and operating results. The Company may from time-to-time desire to exit certain programs or businesses, or to restructure its operations, but may not be successful in doing so. The Company may pursue mergers, acquisitions, investments and joint ventures, which could divert its management’s attention or otherwise disrupt its operations and adversely affect its results of operations. The Company may not be able to convert its pipeline or orders in backlog into revenue. The Company may not manage its growth effectively. Our forecasts and projections are based on assumptions, analyses and internal estimates developed by our management. If these assumptions, analyses or estimates prove to be incorrect or inaccurate, our actual operating results may differ materially from those forecasted or projected. The market adoption of our product is evolving and may develop more slowly or differently than we expect. Our future success depends on the growth and expansion of these markets and our ability to adapt and respond effectively to evolving markets. Interruption or failure of our information technology and communication systems could impact our ability to effectively provide our products and services. We are subject to cybersecurity risks to operational systems, security systems, infrastructure, integrated software in our products and customer data processed by us or third-party vendors or suppliers and any material failure, weakness, interruption, cyber event, incident or breach of security could hinder the effective operation of our business.

Copyright © 2024 QT Imaging, Inc. All Rights Reserved. Risk Factors – Cont. Risks Related to the Business Combination The Proposed Business Combination may disrupt current plans and operations of the Company. If the Proposed Business Combination’s benefits do not meet expectations of investor or securities analysts, the market price of the SPAC’s securities, or following the consummation of the Proposed Business Combination, the combined company’s securities, may decline. The valuation ascribed to the combined company may not be indicative of the price that will prevail in the trading market following the Prosed Business Combination. If an active market for the combined company’s securities develops and continues, the trading price of the combined company’s securities following the Proposed Business Combination could be volatile and subject to wide fluctuations in response to various factors, which could contribute to the loss of all or part of your investment. Both the SPAC and the Company will incur significant transactions costs in connection with the Proposed Business Combination. The SPAC and the Company many not successfully or timely consummate the Proposed Business Combination, including the risk that any required regulatory approvals are not obtained, are delayed or are subject to unanticipated conditions that conde adversely affect the combined company or the expected benefits of the Proposed Business Combination or that the approval of the stockholders of the SPAC is not obtained. The consummation of the Proposed Business Combination is subject to a number of conditions and if those conditions are not satisfied or waived, the Proposed Business Combination agreement may be terminated in accordance with its terms and the Proposed Business Combination may not be completed. There is no guarantee that a stockholder’s decision whether to redeem its shares for a pro rata portion for the trust account will put the stockholder in a better future economic position. Legal proceedings in connection with the Proposed Business Combination, the outcomes of which are uncertain, could delay or prevent the completion of the Proposed Business Combination. Following the consummation of the Proposed Business Combination, the combined company will incur significant increased expenses and administrative burdens as a public company, which could have an adverse effect on its business, financial condition and results of operation. Changes in laws or regulations, or a failure to comply with any laws and regulations, may adversely affect the Company’s or the combined company’s business, including the ability of the parties to consummate the Proposed Business Combination, and results of operation of the Company or the combined company. The ability to successfully effect the Proposed Business Combination and the combined company’s ability to successfully operation the business thereafter will be largely dependent upon the efforts of certain key personnel of the Company, all of whom we expect to say with the combined company following the Proposed Business Combination. The loss of such key personnel could negatively impact he operation and financial results of the combined business. The SPAC’s ability to complete an initial business combination may be adversely affected by downturns in the financial markets or in economic conditions, increases in oil prices, inflation, increases in interest rates, supply chain disruptions, declines in consumer confidence and spending, the ongoing effects of the COVID-19 pandemic, including resurgences and the emergence of new variants, geopolitical instability, such as the military conflict in the Ukraine.

Thank You! 32